Over the past few years, Descemet membrane endothelial transplantation (DMEK) has become the gold standard for treating endothelial corneal diseases. This is a result of numerous innovations that have shaped it into a standardized, minimally invasive procedure. One important milestone was the provision of pre-prepared, precut lamellae (LaMEK) from specialized tissue banks to external transplantation centers. This has enabled more surgeons to benefit from high-quality, quality-assured lamellae.
Through the approval of DMEK RAPID in 2021, the next breakthrough has been reached. Surgeons can now receive pre-prepared DMEK lamellae preloaded in an injector, for direct injection into the eye without further manipulation, enhancing both the safety and ease of DMEK surgery.
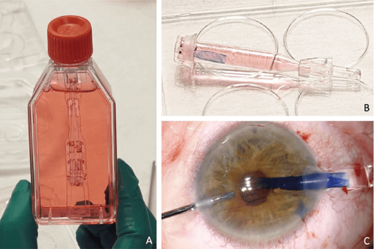
Change in transplant surgery
The revolution of corneal surgery by DMEK can be attributed to its particularly gentle approach, where visual rehabilitation is much faster and more effective than other methods, postoperative follow-up is easier, and rejection rate is lower.
The KHERI Research Institute of the Sulzbach Eye Hospital have been on the forefront of DMEK innovation, with their contributions being pivotal to its success. One example of this is the world’s first DMEK injection system for touchless lamellar corneal transplantation, which they introduced in 2011 and has already been used to perform 60,000 corneal transplantations worldwide (1). Two years later, a new “liquid bubble” technique was introduced, a particularly gentle and safe preparation technique for transplanting donor lamella (2, 3).
Specialized cleanroom tissue bank for lamellar transplants
Since 2016, the Knappschaft Tissue Bank in Sulzbach has been one of the most modern cleanroom facilities in Europe for the production of high-quality corneal transplants. The donor network now consists of 17 German donor hospitals, with the tissue bank having a capacity of 1,000 certified transplants annually. As an official partner of the German Society for Tissue Transplantation, transplants are processed under the highest-quality standards, and are made available to the Sulzbach Eye Clinic and external transplant clinics. In 2021, one in every eight corneal transplants transplanted in Germany came from Sulzbach.
The Knappschaft Tissue Bank particularly specializes in the production of pre-prepared corneal lamellae (Precut-LaMEK). Lamellar grafts require a particularly high manufacturing quality compared to classic full transplants, which the cleanroom tissue bank meets as a result of the entire manufacturing process being subject to a strict, certified quality-assurance system.

Precut LaMEK: a success
Precut is the new trend in DMEK surgery. This is due to the challenging manual preparation process, which has driven more transplant centers to source pre-prepared, precut lamellae to improve safety and quality of grafts. Although experienced anterior segment surgeons learn implantation relatively quickly, manual preparation of donor lamella poses risks. Manual dissection of the lamellae, which are only about 15 µm thick, is usually performed in the operating room shortly before the start of the procedure and can be incorrectly prepared with possible graft loss. Similarly, “fresh” graft preparation in the operating room is neither standardized nor validated.
One solution to this problem is to transfer the production of lamellae to specialized tissue banks. In Germany, only two tissue banks, Sulzbach and Hanover, are approved to produce and market pre-prepared lamellae.
By using pre-prepared lamellae, the surgeon avoids the risk of incorrect preparation and graft loss. With LaMEK, the surgeon receives a pre-prepared lamella that has been quality-controlled with regard to density, morphology, and vitality of the endothelial cells and has been produced in a standardized manufacturing process in a certified cleanroom. The risk of the preparation lies entirely with the manufacturing tissue bank.
This has led to a high acceptance rate. To date, more than 2,000 pre-prepared LaMEK have been produced in Germany, with the preparation success rate being over 95 percent.
How safe are pre-prepared DMEK lamellae?
Comparisons between the long-term effects after DMEK transplantation with Precut-LaMEK and lamellae prepared immediately before surgery showed that after six months, when assessing graft failure, endothelial cell count and visual acuity, there was no significant difference between patients who received pre-prepared grafts and those who received lamellae prepared immediately pre-op. In particular, there was no higher graft failure owing to the use of pre-prepared grafts (4). This demonstrated the safe use of pre-prepared lamellae, as shown in US studies. There was no evidence of a toxic effect of the dextran-containing culture medium (5).
Europe-wide introduction of DMEK RAPID
The Europe-wide approval of DMEK RAPID, as the first preloaded transplantation system for minimally invasive DMEK transplantation, signposted the achievement of the next important step in the evolution of DMEK (6).
For the first time, clinicians have the option of using a pre-prepared and preloaded DMEK lamella which is ready to use. These grafts can be injected directly into the eye without further manipulation, as shown in Figure 3, simplifying transplantation, allowing numerous ophthalmic clinics and external surgeons to perform non-contact, standardized transplantation without risk of dissection. These advantages enhance the safety and ease of DMEK surgery.
The foundation of the DMEK RAPID transplantation system is its patented “ready-to-use” design. Like Precut-LaMEK, the lamella is prepared in advance in the tissue bank but is additionally loaded into the DMEK RAPID injector system and are ready for direct use by the surgeon. The graft does not need to be manipulated or drawn into the injector cartridge; if necessary, the lamellae can be restained within the closed system.
Registration study at the Sulzbach Eye Clinic
A comparison of cell loss and endothelial cell viability after transport between the new DMEK RAPID injection system and Precut-LaMEK (7) showed no significant difference when observed in the viewing chamber. Both groups demonstrated comparable low cell loss, indicating that the transport of preloaded grafts has no negative impact on graft quality and viability.
First pre-prepared DMEK RAPID delivered
In December 2021, the world’s first DMEK RAPID were delivered to German external transplant clinics. The Knappschaft Tissue Bank in Sulzbach is currently the only cornea bank manufacturing and shipping these high-quality, pre-prepared DMEK lamellae under cleanroom conditions, using the liquid bubble technique. The lamellae are then preloaded in approved locations throughout Germany.
The Sulzbach Eye Clinic has used the DMEK RAPID system to treat patients for three years and because of the innovations it has brought to DMEK, the demand from patients and referring physicians has increased dramatically. In 2021, the milestone of 500 transplantations was reached, making the Sulzbach Eye Clinic one of the largest corneal transplant centers in Germany. DMEK RAPID is currently being rolled out across Europe; the approval process is already well-advanced in most European countries, especially Italy and the Netherlands.
Quelle: https://theophthalmologist.com/subspecialties/a-rapid-revolution





